Research Interests
Molecular basis of tumor resistance to apoptosis and οncogenic EMT
We aim to understand the molecular physiology of tumor resistance to apoptosis mediated by conventional anti-neoplastic modules as well as by the endogenous immune-mediated cytotoxicity and delineate the underlying mechanisms. Given that resistant tumors are more likely to acquire invasive and migratory properties and vice versa, we further aim to explore common molecular networks that might orchestrate both tumor resistance to apoptosis and the early metastatic steps, such as the oncogenic epithelial to mesenchymal transition (EMT). On this context, we have identified novel gene products, including the zinc finger transcription factor, Yin Yang 1 (YY1) and the Raf-1 Kinase inhibitory protein (RKIP) and addressed their role as members of molecular ‘circuitries and networks’’ that control tumor immunoescape, chemo-resistance and EMT.
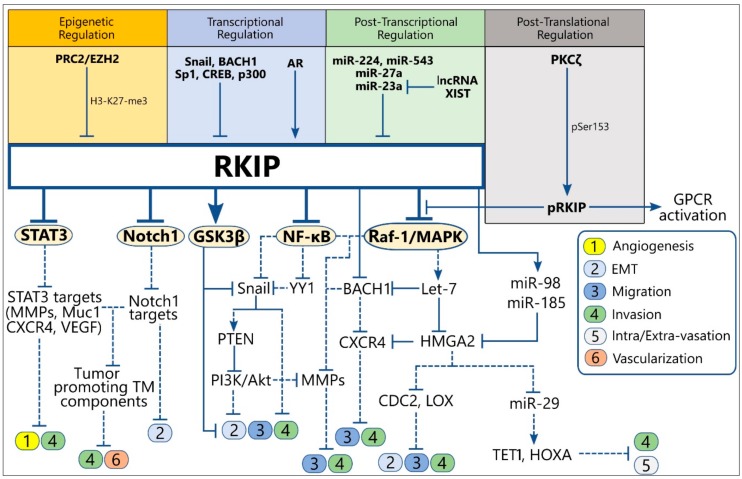
Furthermore, we are interested in exploring the epigenetic regulation of YY1 and RKIP, as well as the clinical significance of newly identified post-translational modifications in the aforementioned genes that have been detected in various solid and hematological malignancies. Our ultimate goal is to translate our findings into new gene signatures characteristic of tumor therapeutic resistance and EMT, thus establishing novel biomarkers with prognostic and/or therapeutic significance on a personalized basis.
LncRNAs and cancer stem cell biology
The existence of cancer stem cells (CSCs) within the bulk of the tumors not only sustains their growth but is also considered to be responsible for promoting metastasis and tumor resistance to conventional chemotherapies. As such, CSCs are highlighted by the experts as the ideal therapeutic targets in most solid tumors, once we understand the molecular mechanisms that regulate their unique biological properties.
Although long non coding RNAs (lncRNAs) have been proposed to play a key role in regulating transcriptional alterations in coding genes associated with malignant transformation and aggressiveness, their involvement in CSC pathophysiology has not been clearly elucidated.
To this end, we are interested in:
- Identifying lncRNAs with functional roles in the biology of CSCs derived by pancreatic ductul adenocarcinoma (PDAC), a malignancy with the worst prognosis among all cancer types. Our approach integrates ‘omics data’ derived from human bio-materials using high throughput technology platforms.
- Examining the molecular targets of lncRNA dysregulation in PCSCs that are associated with PCSC-specific signaling pathways
- Validating the role of identified lncRNAs in sustaining PCSC functions in vivo, in PDAC xenograft and transgenic mouse models.
The translational potential of the above studies is the discovery of novel PCSC-specific biomarkers that can potentially serve as therapeutic targets in pancreatic tumors.
Stress Regulators And Cancer
Although the psychological stress has been extensively reported to be involved in the onset and progression of many diseases, including cancer, the underlying molecular mechanisms of action remain unknown for the most part. Key regulators of stress response in humans such as the CRH family of neuropeptides are widely known to be involved in regulating chronic inflammation, one of the predisposing factors for oncogenesis and disease progression. However the molecular link among stress, inflammation and malignancy, has not been clearly elucidated.
We are interested in exploring the impact of CRH family members with established role in the regulation of intestinal inflammatory responses, in the growth, metastasis and immunoresistance of colorectal cancer (CRC).
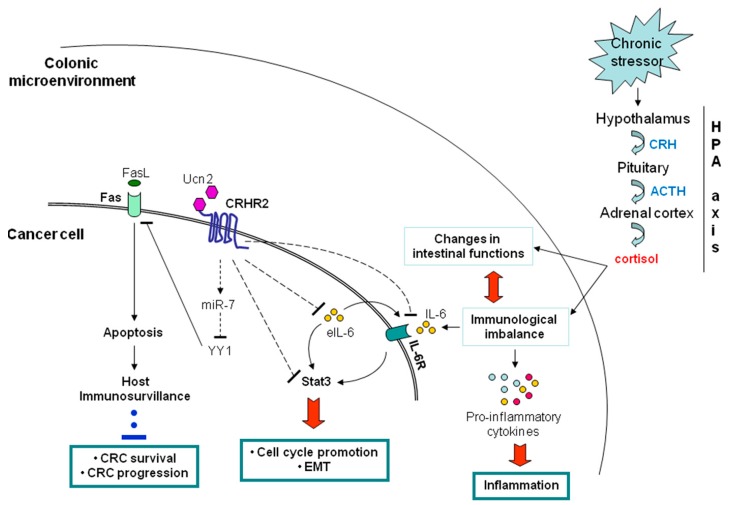
In recent in vitro and in vivo studies we have shown the pattern of expression and activation of CRHR2 receptor in the colon plays a critical role in CRC development and aggressiveness, including tumor immunoescape, by regulating molecular circuits involved in sustaining inflammation in the gut. We continue to address questions related to :
- The role of CRHR2 in CRC resistance to conventional chemotherapy
- The role of CRHR2 in the pathophysiology of intestinal inflammation
- The epigenetic regulation of CRHR2 and other CRH family members in various malignancies.
Our ultimate goal is to identify stress regulators with established role in CRC pathophysiology that can benefit tumor prognosis of unresponsive tumor types and serve as novel therapeutic targets.
Drug screening and development
in cancer era
We are interested in testing the anti-neoplastic activities of novel chemo- and immunotherapeutics in pre-clinical models of various solid and hematological malignancies, and examine the underlying molecular mechanisms of action. We have been participating in identifying the role of anti-CD80s (Galiximab) and anti-CD20 mAbs (LFB-603) as well as of BH3-only protein mimentic obatoclax (GX15-070) as putative co-sensitizing and pro-apoptotic agents in Rituximab resistant B-NHL models, while the novel proteasome inhibitor NPI-0052 (Marizomib) was found to have broad sensitizing and anti-metastatic activities in prostate cancer and other solid tumors. Most of the above drugs are already in Phase 1 and/or 2 clinical trials as a monotherapy or part of a combined therapy in patients with advanced malignancies including relapsed/refractory multiple myeloma and B-NHL.
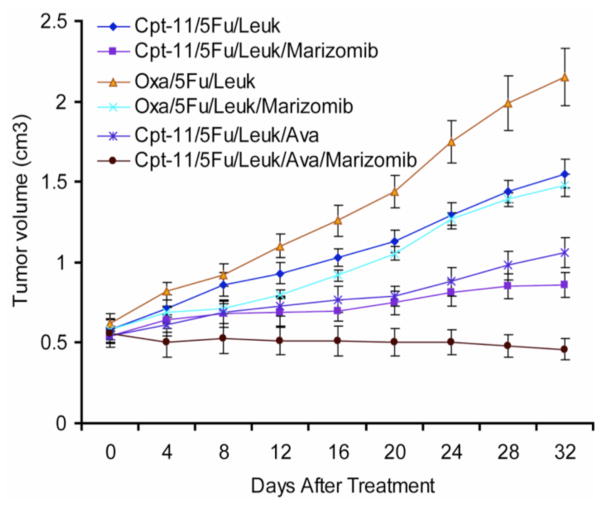
In collaboration with our research partners we are further developing projects based on the application of highthroughput technology systems that along with ‘omics profiling and biomarker development in cancer can be used in drug screening and discovery. On this context we are participating in building up an integrated small and large molecule high throughput screening automated platform for identifying, within FDA approved drug libraries, biologically active molecules with anti-tumor activities. Among the drug targets of our interest are also the PCSCs, as stated above.
